Investors and partners
Molecular biological tests based on epigenetic biomarkers that enable early, reliable and rapid cancer diagnostics – that is the goal of oncgnostics GmbH. For studies, projects or international sales we collaborate with various partners.
About us
The management of oncgnostics GmbH consists of the founding members of the company. A highly motivated and constantly growing team of industry-experienced employees and ambitious scientists work at oncgnostics GmbH.
Products
GynTect® supports the early detection of cervical cancer. In addition, oncgnostics GmbH develops molecular biological cancer tests for head and neck tumours and ovarian cancer and is working on further research projects.
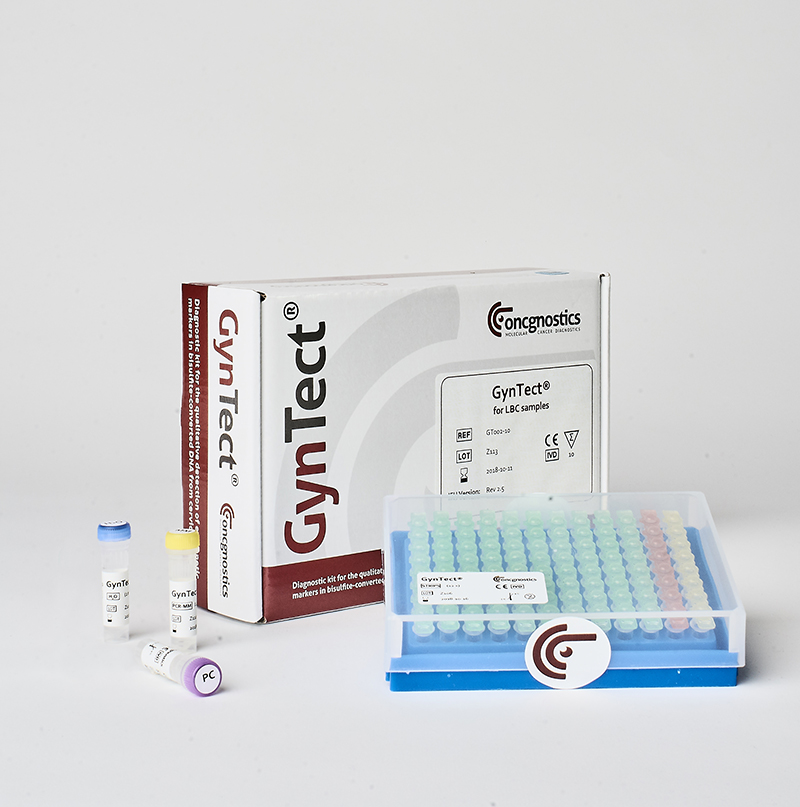
GynTect® – the molecular biological test for cervical cancer
Detailed information on GynTect®, our molecular biological test for cervical cancer, is available at
In addition to a special section for laboratories and gynaecologists, patients can also find interesting information about cervical cancer and cervical cancer screening.
News und Aktuelles
Certain projects in our company are co-financed by the European Union with ERDF and ESF funds and the Free State of Thuringia (Thuringian Ministry of Economic Affairs, Science and Digital Society).







 oncgnostics GmbH
oncgnostics GmbH Shaily / shutterstock
Shaily / shutterstock



