Products: Cancer tests based on epigenetic biomarkers
Our products are cancer tests that detect characteristic epigenetic changes, i.e. DNA methylations, in cancer cells. These highly informative biomarkers, which we have identified and patented, are the basis of our research and development for further IVD tests.
Our goal is to develop tests for the diagnosis of cancer that are rapid, safe and non-invasive in application.
GynTect® – assay for cervical cancer screening
GynTect® enables the simple and reliable detection of methylated DNA regions in cervical scrapes. Methylation of GynTect® biomarkers occurs specifically in cervical cancer or its precursors, but not in samples from healthy women. Thus, GynTect® can help to clarify whether there is already a tissue alteration that needs to be treated.
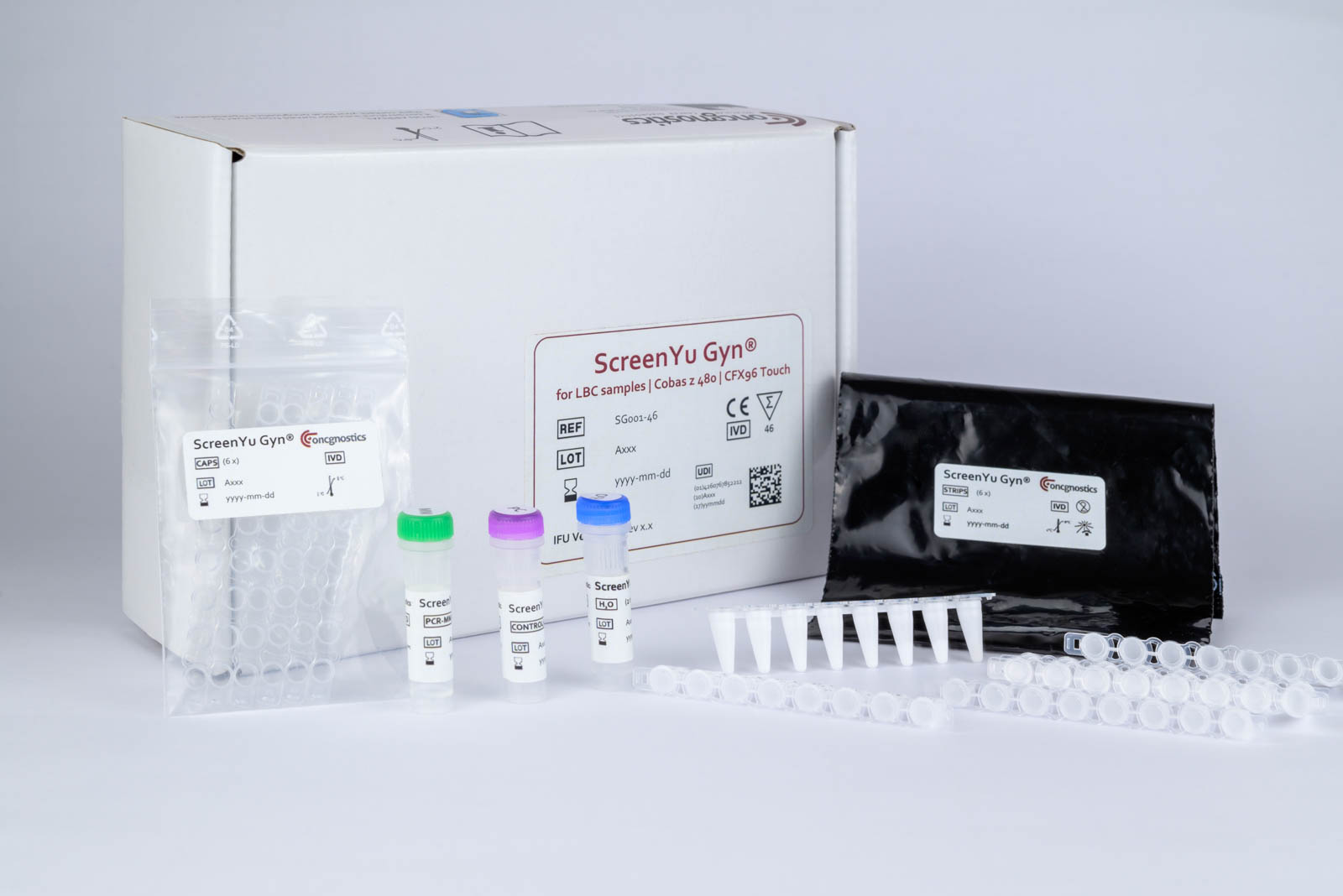
ScreenYu Gyn® – the first step to cervical cancer screening
ScreenYu Gyn® is the second diagnostic test for the field of cervical cancer screening from oncgnostics GmbH. The molecular biological cancer test is derivative of GynTect®. For ScreenYu Gyn® only one biomarker, i.e. a methylated DNA region, is detected in cervical smear samples together with a control marker. Thus, only one PCR reaction is required per swab sample. The assay serves as the basis for the development of a test for use in screening.
Pipepline Products
Diagnostics of head and neck tumors
Worldwide, head and neck tumors are among the five most common cancers. In Germany, approximately 17,000 people are diagnosed with head and neck cancer each year. Head and neck tumors can often develop unnoticed for a long time, as it is difficult for those affected to recognize the signs themselves. However, the later the tumors are detected, the lower the chance of cure. Early diagnosis is therefore very important. The diagnosis of such a head and neck tumor by a specialist is not easy, because there are no early detection tests so far and accordingly no screening.
We are developing tests based on DNA methylation markers that detect cancer cells in the patient’s saliva. Our vision is to contribute significantly to a regulated screening program for head and neck cancer in the future.
Automated Screening Assay for Cervical Cancer
While GynTect is used for clarification as part of organized cervical cancer screening, we are now developing a test that can prospectively be used directly in primary screening. A first step in this direction is the molecular biological test ScreenYu Gyn®, which has received CE IVD approval in May 2022. Thanks to only one biomarker, this test is cheaper to produce, easier to perform and easier to automate. In the future, we would like to use a screening assay derived from this test primarily to serve countries and regions where cervical cancer screening has hardly taken place to date, for example because the infrastructure requires a low-threshold offering. After all, most of the women newly diagnosed with cervical cancer each year live in these countries.