oncgnostics at Medica 2022:
-
Cervical cancer screening test conquers China
-
Establishing early detection of head and neck tumours
-
First study results for screening test for vulva and vaginal cancer
-
At the joint stand of medways e.V. in Hall 15, Stand K10
Jena / Düsseldorf, November 08, 2022 – oncgnostics GmbH will present its technology, products and current study results at the largest trade fair in the medical sector. The company is an expert in molecular cancer diagnostics. Life-saving early detection is possible with the cancer tests developed by oncgnostics, which are based on patented biomarks.
Cancer diagnostics through biotechnology
DNA methylations form the basis of oncgnostics’ work. Specific changes in the DNA methylation pattern occur when cancer develops or is already present. The tests from oncgnostics detect these changes.
Innovation in cervical cancer screening
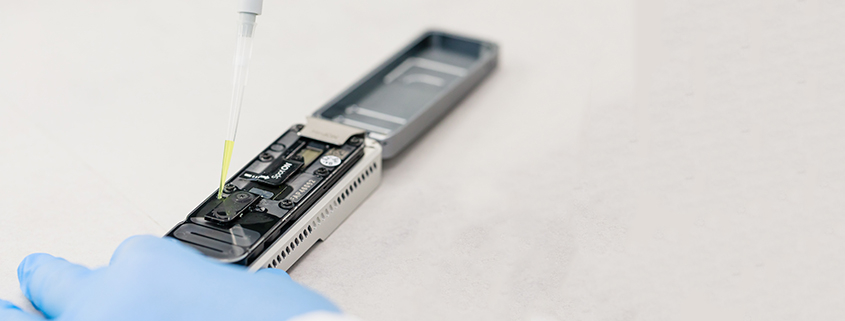
GynTect is the company’s first product. Used in cervical cancer diagnostics, the test is already being marketed in several European countries. In August of this year, it was approved in China, where it is marketed under exclusive licence. It is the first methylation test for triage of HPV-positive cases on the Chinese market.
The methylation test in cervical cancer screening
A cervical smear, as it is also taken for thin-layer cytology or the HPV test, is sufficient for the procedure. The test is a decision-making aid on how best to proceed after an abnormal screening result. GynTect not only allows to detect whether a tumour is already present, but also detects cervical lesions that may develop into cervical cancer – years in advance. Thus, risk assessment is greatly facilitated: on the one hand, unnecessary, premature operations can be avoided, and on the other hand, the chance for early and thus promising therapeutic measures can be increased.
ScreenYu Gyn is a further development of GynTect. The diagnostic test, which was CE IVD-approved in May this year, uses only one methylated DNA region, whereas GynTect detects six methylated DNA regions (biomarkers). This makes it particularly well suited for automation. With a simplified and automated test version, countries and regions can be served in which cervical cancer screening has hardly taken place so far. Most new cases of cervical cancer and most deaths due to the disease occur in these countries.
Establish early detection for head and neck tumours
Head and neck tumours are among the five most common cancers worldwide. They often develop unnoticed for a long time because the symptoms are usually unspecific for those affected. So far, no early diagnosis has been established for this group of tumours. oncgnostics is developing a test to change this. In this test, DNA methylation markers identified in cancer tissue and validated by oncgnostics, are detected in the patient’s saliva. The test will initially be used in post-surgical follow-up, as tumour markers that already appear in the primary tumour are also detectable in re-emerging tumours.
Vulva and vaginal cancer: early detection also overdue
Early detection of vulvar and vaginal cancer is also difficult due to non-specific symptoms or no symptoms at all. Initial studies have confirmed that a methylation test with the same biomarkers as for cervical cancer may also be useful here. oncgnostics is continuing to work on diagnostics for the early detection of vulvar and vaginal cancer on this basis.
_____________________________________________
About oncgnostics GmbH:
Jena-based oncgnostics GmbH specialises in the early detection of cancer. Its tests detect changes that are characteristic of the DNA of cancer cells. The company, founded in 2012, launched GynTect in 2015. In the context of early cervical cancer detection, the test clarifies whether cervical cancer or precancerous lesions are already present. oncgnostics GmbH is also conducting research into screening tests for other types of cancer. Detailed information is available at www.oncgnostics.com/en.
Free image material: www.oncgnostics.com/en/downloads
Contact:
oncgnostics GmbH
Löbstedter Str. 41
07749 Jena
Germany
Phone: +493641/5548550


 Studio Beetz
Studio Beetz Andrey_Popov/Shutterstock.com
Andrey_Popov/Shutterstock.com





